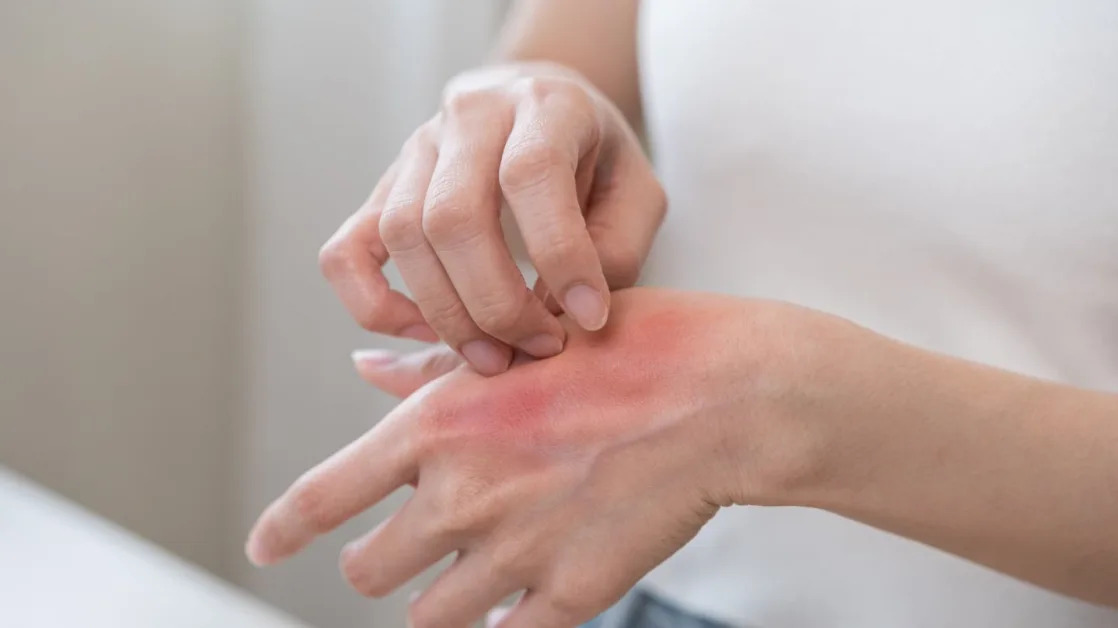
LEO Pharma has gained marketing authorisation from the UK's Medicines and Healthcare Products Regulatory Agency (MHRA) for its topical pan-Janus kinase (JAK) inhibitor, Anzupgo (delgocitinib) 20mg/g cream, to treat chronic hand eczema (CHE) in Britain.
The approval enables the treatment of adults with moderate to severe CHE when topical corticosteroids are inadequate or inappropriate.
The decision was based on the positive outcomes of the Phase III programme, which encompassed the DELTA 1 and DELTA 2 trials. The trials assessed the cream's efficacy and safety against a cream vehicle, meeting their primary and secondary goals.
Subjects from the DELTA 1 and DELTA 2 trials were given the opportunity to join the DELTA 3 trial, a 36-week open-label extension study.
LEO Pharma UK and Ireland vice-president and general manager Leanne Walsh stated: “Today’s MHRA approval of delgocitinib cream marks a significant milestone for adults in Great Britain living with moderate to severe chronic hand eczema.
“This approval offers a new treatment paradigm and demonstrates our commitment to addressing the unmet needs of people living with skin conditions.”
The cream targets the JAK-STAT signalling pathway involved in CHE pathogenesis. The condition is marked by skin barrier dysfunction, inflammation and changes in the skin microbiome.
The current approval of delgocitinib cream adds to its existing authorisations in the European Union and Switzerland for the same indication.
LEO Pharma obtained exclusive development and commercialisation rights to the cream for dermatological uses globally, excluding Japan, through a license agreement with Japan Tobacco (JT) in 2014. JT holds the rights in Japan.
In September 2024, the European Commission (EC) granted marketing authorisation for delgocitinib cream to treat adults with moderate to severe CHE.
LEO Pharma UK and Ireland is working with the National Institute for Health and Care Excellence (NICE) to ensure the cream's availability on the National Health Service (NHS).
Chronic hand eczema is a complex and variable inflammatory skin condition, with primary symptoms such as itching and pain affecting the hands and wrists.
"LEO Pharma’s eczema cream gains MHRA marketing authorisation" was originally created and published by Pharmaceutical Technology , a GlobalData owned brand.
The information on this site has been included in good faith for general informational purposes only. It is not intended to amount to advice on which you should rely, and we give no representation, warranty or guarantee, whether express or implied as to its accuracy or completeness. You must obtain professional or specialist advice before taking, or refraining from, any action on the basis of the content on our site.