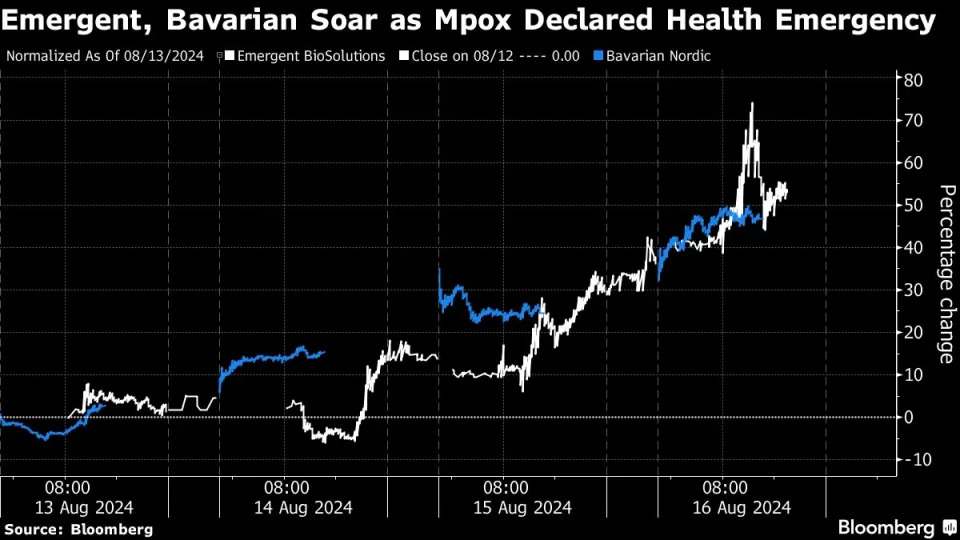
(Bloomberg) -- Emergent BioSolutions Inc. led an advance in the stocks of companies working on vaccines and antiviral products this week as investors await word from US regulators on if its smallpox vaccine can also be used for the fast-spreading mpox virus.
The vaccine maker — which has said it expects US Food and Drug Administration approval by the end of this quarter — climbed more than 60% over the past four sessions with the World Health Organization declaring the mpox outbreak in Africa a global health emergency on Wednesday. The stock has seen its value more than quadruple in 2024.
Other drug developers in the space also saw interest in their stocks surge. Past outbreaks have spurred similar volatility as daytraders look for potential winners despite the many roadblocks to advances that could help in the fight against the spread of a new virus variant.
Bavarian Nordic A/S — the only company with a vaccine approved for mpox in the US and Europe — extended its rally on Friday after the drugmaker said it is seeking to get its shot approved for adolescents. Bavarian shares have gained in nine straight sessions, adding 18% on Friday in Copenhagen trading, on expectations that sales of its vaccine will surge.
Siga Technologies Inc., which makes a smallpox treatment called Tpoxx that’s being studied in patients with mpox, shot up 24% on Friday before paring gains to 9%. The company, which is backed by billionaire Ron Perelman, said on Thursday that an mpox study of the treatment failed to meet its main goal but the drug showed some benefits.
Companies may face difficulties offsetting low-cost sales and donations to poor nations since the mpox vaccine isn’t currently in high demand in wealthier countries. While the disease risk for the general population in Europe remains low, the European disease prevention agency said on Friday that they expect more imported cases of the new mpox strain will appear in Europe in the coming weeks.
Jean Kaseya, who heads Africa Centres for Disease Control and Prevention, has been vocal about the obstacles facing a vaccine rollout. Many countries can’t afford the vaccines that are currently available at $100/dose and require two doses, Kaseya said. The continent is estimated to need $4 billion to fight the disease.
Benchmark analyst Robert Wasserman, who holds the lone buy rating on Emergent, is optimistic its smallpox shot, ACAM2000, could be used for mpox.
“An FDA approval followed by a procurement order from the US for the stockpile or from international agencies could provide a boost for Emergent’s medical countermeasures segment, ” Wasserman wrote in a note dated Thursday.
Tonix Pharmaceuticals Holding Corp. initially climbed 20% after its chief executive officer touted its efforts to advance a new mpox vaccine into human trials on Bloomberg Television before closing 2.1% lower.
Microcap biotech GeoVax Labs Inc., which has an experimental mpox vaccine more than doubled on Friday for its best one-day gain in more than two years.
(Updates share price moves throughout.)